WAEC GCE Chemistry Alternative to Practical Questions and Answers 2023 is now Available.
The Waec Gce chemistry practical 2022 alternative questions and answers for exam have been released. The General Certificate Examination (GCE) for WAEC Chemistry alternative to practical paper will be written on Tuesday, 12th December, 2023 from 2:00 pm to 3:30 pm.
We made this post to highlight samples of the Specimens, alternatives to practical graphs, and calculations that were used in the Chem Practical Examination past questions and syllabus.
Alternative WAEC GCE Chem Practical 2023.
Paper 3 (Alternative To Practical)
Answer Three questions.
Write your answers in the spaces provided.
1. A student adds a known mass of magnesium ribbon to 100 cm3 of dilute hydrochloric acid (an excess) in the apparatus shown below. Hydrogen gas is evolved. The time taken to collect 48 cm3 of gas at room temperature and pressure is measured.
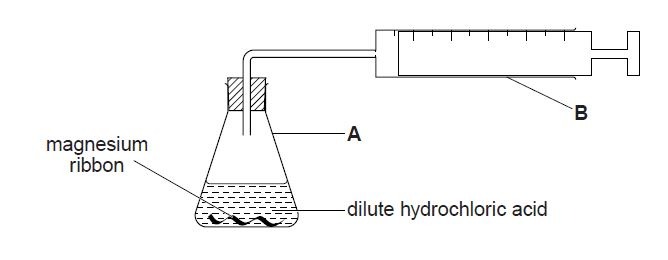
(a) (i) Name apparatus A.
(ii) Name apparatus B.
(b) (i) Calculate the number of moles of hydrogen in the 48 cm3 of gas.
[1 mole of any gas occupies 24 000 cm3 at room temperature and pressure.]
(ii) Use the equation below to deduce the mass of magnesium used in the experiment to produce 48 cm3 of hydrogen at room temperature and pressure. [Ar : Mg, 24]
Mg + 2HCl MgCl2 + H2
(c) Give a test for hydrogen gas.
(d) What is the effect on the time taken to collect the same volume of gas if,
(i) large lumps of the same mass of magnesium are used instead of magnesium ribbon,
(ii) the reaction is carried out at a higher temperature?
2. (a) A student investigates the electrolysis of aqueous copper(II) sulfate using graphite electrodes and the apparatus shown below.
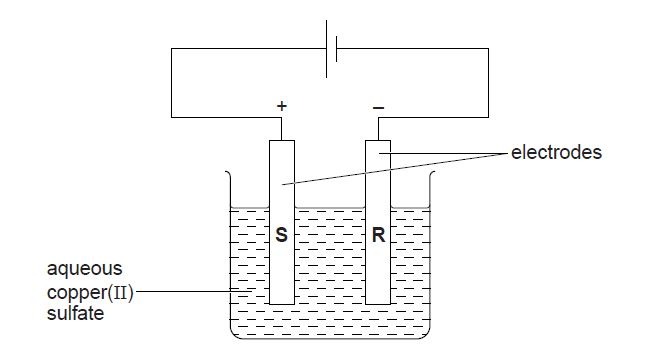
(i) What is observed at electrode R?
(ii) Construct the equation for the reaction taking place at electrode R.
(iii) When graphite electrodes are used a colour change is seen in the solution. What is this colour change? Explain why it happens.
(iv) When graphite electrodes are used, a gas is evolved at one of the electrodes. Name the gas and give a test for the gas.
(v) If the graphite electrodes are replaced with copper electrodes, no colour change is seen on the electrolysis of the solution. Explain why.
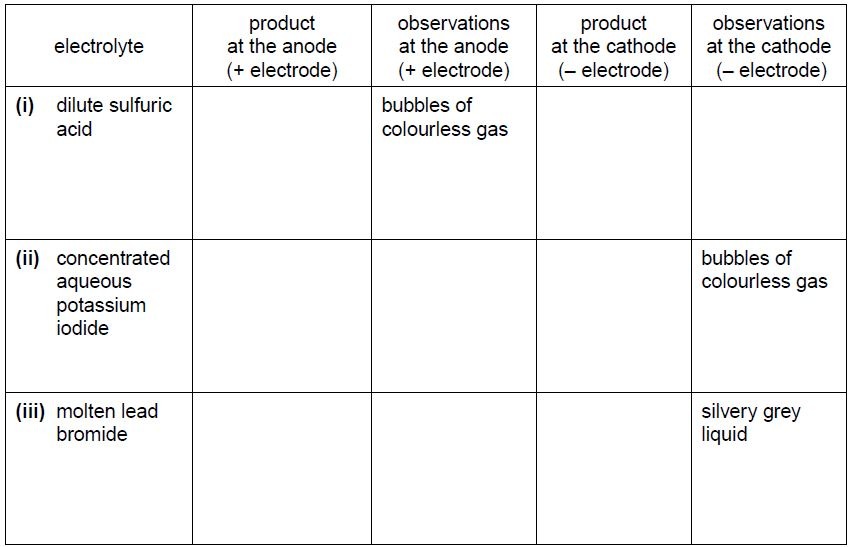
(b) The student does more experiments using the apparatus in (a) with graphite electrodes
but, in each case, using a different electrolyte.
Complete the table below.
The details of the WAEC Chem practical drawings and answers will be made available to you candidates as soon as it is released. Keep following this page and make sure you bookmark this site for reference purposes.
If you have any questions, endeavour to use the comment box below…

Answers please
Please can I get the 2021 own
Please,where are the corresponding answers? Thank you!
Go and read and stop finding answers
Tah want do u know
Please which year is this?
Please where can I get answers from
Please where are the answer to the questions.
Please where are the answers
to the questions?
Intresting questions
Where is volumetric analysis
No Titration?
This is for GCE. For school exam, WAEC Chemistry Practical Questions
Is this 2022
What about de answer
Question and ans for chemistry alternative to practical
I really hope this information is reliable…….
hope the question is correct?
and why no titration?
What about the solutions
Its only two questions I see there is no number 3??
thank you for noticing this I was just about to say that
Yeah, that’s true…
Why is there no Titration this year?
Also, the 3rd question is missing…
Question and ans for chemistry alternative to practical
But the practicals I no see Titration and Qualitative analysis questions, why?
Can i join any of their WhatsApp group ?
Please how reliable is this information?
pls i hope this is the original pratical questions