WAEC Chemistry Practical Answers 2025 Released – See Chemistry Specimen Here.
The Waec chemistry practical 2025 specimen paper for SSCE will now be written on Thursday, 16th May 2024 from 09:30 hrs. to 11:30 hrs. for the first set and from 12:00 hrs. to 14:00 hrs. for the second set. The paper is Waec Chemistry Practical Alternative A.
We made this post to show apparatus, Waec chemistry practical specimens, graphs, and calculations that are used in the Chemistry Practical Examination questions and syllabus.
Here, we will be providing you with the Waec Chemistry practical 2024 specimen questions and materials that will be used for Examination preparation. Read the answers below.
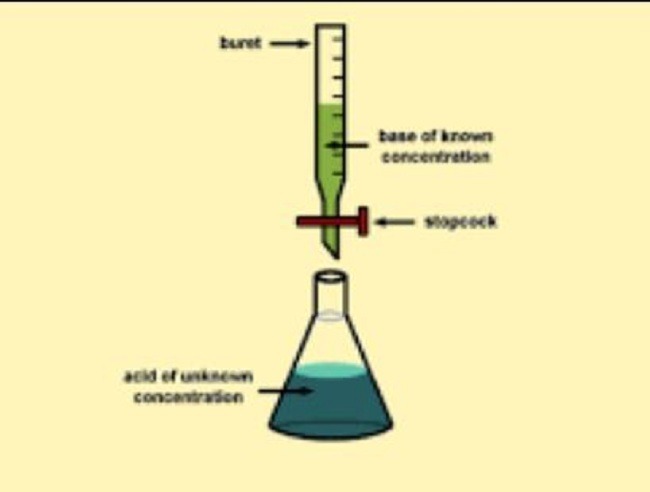
WAEC Chemistry Practical Specimen 2024.
The following apparatus and materials will be required by each candidate for Waec Chemistry Practical 2024;
(a) one burette of 50cm³ capacity.
(b) one pipette, either 20cm³ or 25cm³.
(c) the usual apparatus for titration.
(d) the usual apparatus and reagents for qualitative work including the following with all reagents appropriately labelled;
(i) dilute sodium hydroxide solution.
(ii) dilute hydrochloric acid.
(iii) dilute trioxonitrate(v) acid
(iv) silver trioxonitrate(v) solution.
(v) acidified potassium dichromate solution.
(vi) aqueous ammonia.
(vii) lime water.
(viii) red and blue litmus paper.
(ix) dilute tetraoxosulphate(vi) acid.
(e) Spatula
(f) filtration apparatus.
(g) one beaker
(h) one boiling tube
(i) four test tubes.
(j) Methyl orange as an indicator.
(k) mathematical table/calculator
(l) wash bottle containing distilled/deionized water.
(m) a burning splint
(n) watch glass
(o) bunsen burner/source of heat
(p) droppers.
Each candidate should be supplied with the following where n is the candidate’s serial number.
(a) 150cm₃ of a solution of HCL in a corked flask or bottle labeled ‘An’. These should all be the same containing 8.5cm³ of conc HCL per dm₃ solution.
(b) 150cm₃ of Na²Co³. 10 H2O in a corked flask or bottle labeled ‘Bn’. These should all be the same containing 5g of hydrated salt per dm₃ of solution.
(c) One spatulaful of glucose in a specimen bottle labelled ‘Cn’. This must be the same for all candidates.
WAEC Chemistry Practical Questions & Answers.
1. A is 0.100 mol dm-3 solution of an acid. B is a solution NaOH containing 2.8 g per 500 cm3.
(a) Put A into the burette and titrate it against 25.0 cm3 portions B using methyl orange as an indicator. Repeat the titration to obtain consistent titres. Tabulate your readings and calculate the average volume of A used.
(b) From your results and the information provided above, calculate the:
(i) number of moles of acid in the average titre;
(ii) number of moles of NaOH in the volume of B pipetted;
(iii) mole ratio of acid to base in the reaction
[H = 1.00, O = 16.0, K = 39.0]
ANS: (a) titration answer soon.
(b) (i) number of moles of acid = 0.100 x VA
1000
= X mole(s) [3sig. Fig to score]
1000cm3 contains 0.100 mole(s)
VA will contain 0.100 x VA
1000
= X moles [3 Sig. Fig. to score]
(ii) Number of moles of NaOH in B
500cm3 of B contains 2.8g of NaOH
1000cm3 of B will contain 2.8 x 1000 = 5.6 NaOH
500
Molar mass of NaOH = 39 + 16 + 1 or 56 gmol-1
Conc of B = 5.6 = 0.100 mol dm-3
56
(iii) Mole ratio of acid to base = X: Y to nearest whole number ratio.
2. C is a mixture of two salts. Carry out the following exercises on C. Record your observations and identify any gas(es) evolved. State the conclusion drawn from the result of each test.
(a) Put all of C into a boiling tube and add about 5cm3 of distilled water. Stir thoroughly and filter. Keep both the residue and the filtrate.
(b) To about 2 cm3 of the filtrate, add few drops of K2Cr2O7/H+. Boil the mixture and then allow to cool as (D).
(c) (i) Put the residue in a test tube and add dilute NaOH(aq). Shake the mixture and divide the solution into two portions.
(ii) To the first portion from (c)(i), add NaOH(aq) in drops and then in excess.
(d) What organic class does C belong to?
ANS:
|
TEST |
OBSERVATION |
INFERENCE |
|
a) C+ burning splint |
C burns when ignited with a burning splint. |
C is flammable. |
|
b) (i) C + Water |
C forms a solution with water. |
C is a mixture with water. |
|
(ii) C + K2Cr2O7/H+ve + Heat |
No visible reaction. The K2Cr2O7/H+ve changes colour to green. |
C is a reducing agent. |
|
c) Residue from b (D) + C + NaOH(aq) |
D dissolves to form a reddish-brown solution. A yellowish precipitate is formed and an antiseptic smell is given out. |
D is a solution in D Ethanol, ethanal or a secondary alkanol present. |
|
d) C |
|
C belongs to Alkanols |
3. (a) Explain briefly the observations in each of the following processes:
(i) when carbon(IV) oxide is bubbled through lime water, it turns milky but the milkiness disappears when the gas is bubbled for a long time;
(ii) a precipitate of calcium hydroxide is insoluble in excess sodium hydroxide solution whereas that of lead (II) hydroxide is soluble.
(b) (i) What is a primary standard solution?
(ii) Calculate the mass of sodium trioxocarbonate(V) required to prepare 250 cm3 of 0.15 mol dm-3 solution.
[Na = 23.0; O = 16.0; C = 12.0]
(c) Name one gas that can be collected by:
(i) upward displacement of air;
(ii) downward displacement of air.
ANS: (a) (i): Insoluble CaCO3 formation is responsible for the milkiness produce when CO2 is bubbled through lime water while the disappearance of milkiness is due to the formation of soluble Ca(HCO3)2.
Lime water turns milky with CO2 because CaCO3/ CaCO3(s) is formed. Milkiness disappear when excess CO2 reacts with CaCO3 in water medium forming the soluble Ca(HCO3)2/ Ca(HCO3)2 (aq).
(ii) Calcium hydroxide is not amphoteric. Does not react with an alkali NaOH whereas lead (II) hydroxide is amphoteric so reacts with excess NaOH.
(b) (i) Primary standard solution is one whose concentration is known and can be used to standardize another solution.
(ii) M (Na2CO3) = 106 gmol-1
m(Na2CO3) = C x M x V
= 0. 15 x 106 x 0. 25
= 3. 98 g
(c) (i) Carbon(IV) oxide, sulphur (IV) oxide, hydrogen chloride, oxygen, nitrogen (IV) oxide, chlorine, hydrogen sulphide.
(ii) Ammonia, oxygen, hydrogen, methane.
Bookmark and keep following this page for more updates. Also, ask your questions by posting a comment bellow.
PS: Once again, there is nothing like waec chemistry expo. Do not fall victim to scammers online trying to obtain money from you with fake promises of having access to live question paper before the exam. What we have on this page are likely exam questions from waec past questions and answers to serve as a revision guide.

i need biology practical questions and answers
Do not be deceived. Read and God of waec will help you
Thanks for the guide, please don’t ever rely on make
Findings for yourself
Thank you
Please is this the real answers
Is this a true solution to chemistry practical for tomorrow
NO, Stay there dey play!!!!
Are this the real practical. Thank you very much
Thanks for the revision
🤔
I don’t think so
is this really the question and answer
i do not think so
Please I need chemistry practical questions.
Are we having chemistry tomorrow
Yeah we are writing chemistry practical tomorrow.
Yes
No it was yesterday
no, it is on saturday, question
Which practical are we having tomorrow and name
Is this a real answer
Pls i need physics practical question and answer for 2023
Somewhat real…
Ode
Nothing like that they😂😂😂😂
I’m still confused
Yeah ur right 👍
Please does this site give real questions?.
Pls if it’s real tell us and atop posting fake answers.jor
Thanks for d revision
Must you rely on the answer? Can’t you read your books and leave the remaining to God u will now have a good results thar you can’t defend in higher institutions
brilliant star u think sey u fit write waec on your own and u dey sure say u no go faill
people that did F.math knows what am saying. Read oo but no dull yourself when expo
Is this real or fake
Am not sure oooh
It is not real. Only the questions are correct but the answers? NAH most of them are wrong
Why
Please I need chemistry practice questions
Please I need chemistry practice answer
When you no get sense only the wise will know how they set this question
Is the correct one
I have proof
Pleaase is this real
Not really
Please is this real
Nope…it’s wrong
3a(ii)
Your answer is wrong . Lead hydroxide is soluble in excess sodium hydroxide because it forms a complex Na2Pb(OH)4 not because it is amphoteric.
Hi
Are answers correct? Pls.
There’s no need for the answers to be correct just study it and get the correct answer it’s just to put us through
Then what are you doing in this site.
I noticed it too,slth the instructions and questions seem real I am not dependent on the answers,I will solve them myself
Yes I support you
Please is dis 4real Biko
Well done and remain blessed.
Which prank
May God help us.
But is the real answer
Thanks
This will make us more prepared
Of course
Thanks for the revision.thanks alots god bless u and use
I need answers
It is nice at least it will help us to get more prepared.
Wait is this real or fake
I’m not sure this is real answers
Solve it and get the real answer
This one is just to put us through
No
thursday will determine it
Thanks so much
Thank you very much
Send me white paper for physics practical 2021
Please can you send me white paper for practical chemistry 2021??
Please I need the answer in my email
Dey play 😂😂😂😂
Thanks
Pls what year I’d this solution
2021
Don’t trust in everything
Thanks very much
is it correct
Is the answer up there now correct
In God we trust
Am not sure but some things re similar
All the answer are real… may God help us and bless you.
PLS CAN YOU SEND THE THEORY TO MY EMAIL?
How am I sure
Thanks 4 d assistance we at happy very happy 4 ur favor dir thanks
is it true
How true is iit
Thanks a lot
Aw true is this question
FAKE QUESTION
WAS GOTTEN FROM PREVIOUS YR
Your titration not the same as the acid and base we have in the specimen..so don’t mislead ppl
What do you have as Acid and Base specimen?
wat is the source and how real is the question
Please can u send me the chemistry practical paper?
I’m
DAT TRUE
Yes
Olu! answer na… what do we have in the specimen as an Acid and Base?
You don’t know the answer that’s all and am advising to use this one before you get F9.
Thanks I love this
Is this real
FAKE
I don’t believe
Not real,,it’s obvious
Thanks for the information
Pls give the right thing don’t miss lead pple
Yes
This is real
why should we believe that it is the real questions?
U guys are doing a great job thank you
Do you think is real
THIS IS GOOD SEND TO ME NECO MORE ON MY EMAIL
Are u sure it is fake