See 2023 NECO Chemistry Answers & Questions Here.
The Neco chemistry answers for 2023 questions can now be seen here. The National Examination Council, NECO Chemistry SSCE paper is scheduled to be written on Monday, 24th July 2023 from 10:00 am to 1:00 pm.
This NECO Chemistry questions paper is for Papers III & II: Objective & Essay and will take a total of 3hrs to write.
Here, we will be posting the neco chemistry questions for candidates that will participate in the examination. Note that below is the questions from past questions and answers that we feel are likely questions for SSCE preparation.

NECO Chemistry Answers 2023.
1. a) (i) What is the structure of the atom as proposed by Rutherford?
(ii) Distinguish between the atomic number and the mass number of an element.
(iii) Explain briefly why the relative atomic mass of chlorine is not a whole number.
b) (i) What is meant by first ionization energy?
(ii) List three properties of electrovalent compounds
(iii) Consider the following pairs of elements: 9F and 17CL; 12Mg and 20Ca.
2. a) (i) Define nuclear fission.
(ii) Consider the equilibrium reaction represented by the following equation: A2(g) + 3B2(g) 2AB3(g); H = + kJmol-1. Explain briefly the effect of each of the following changes on the equilibrium composition:
- increase in the concentration of B;
- decrease in pressure of the system;
- addition of catalyst.
b) The lattice energies of three sodium halides are as follows:
|
Compound |
NaF |
NaBr |
NaI |
| Lattice energy/kJmol-1 | 890 | 719 | 670 |
Explain briefly the trend.
c) State the property exhibited by nitrogen (IV) oxide in each of the following reactions:
(i) 4Cu + 2NO2 4CuO + N2;
(ii) H2O+ 2NO2 HNO3 + HNO2.
3. a) (i) Define saturated solution.
(ii) Distinguish between dative bond and covalent bond.
(iii) Explain why sugar and common salt do not conduct electricity in the solid state.
(iii) State the type of intermolecular forces present in: hydrogen fluoride; argon.
(iv) Consider the compounds with the following structures:
S – H —-N and 0 – H —–N
In which of the compounds is the hydrogen bond stronger? Give reason for your answer.
(b) (i) State Dalton’s Law of Partial Pressure.
(ii) If 200cm3 of carbon(IV) oxide were collected over water at 18°C and 700 mmHg, determine the volume of the dry gas at s.t.p.
[ standard vapour pressure of water at 18°C = 15 mmHg]
4. a) (i) Define ionic bond.
(ii) What type of bond(s) exist(s) in: magnesium oxide; ammonium ion?
b) (i) Determine the oxidation number of sulphur in Na2S2O3.
(ii) State Faraday’s first law.
(iii) Give one example each of: acid salt; base salt.
c) (i) Name the type of energy change that occurs in each of the following processes;
I2(s) ———> I2(g);
C1(g) + e- ——> C1-(g).
(ii) State the effect of each of the following aqueous solutions on litmus paper:
Na2SO4(aq);
AlC13(aq)
(iii) Define the term efflorescence.
(iv) Give two uses of activated charcoal.
d) (i) State one use of each of the following processes in the chemical industry: hydrogenation of vegetable oil; cracking; esterification.
(ii) Calculate the amount of silver deposited in moles when 10920 coulombs of electricity is passed through a solution of a silver salt.
[Faraday constant = 96500 C mol-1]
5. a) (i) Define in terms of electron transfer
I. oxidizing agent;
II. reducing agent.
(ii) Write a balanced equation to show that carbon is a reducing agent.
(iii) State the change in oxidation number of the specie that reacted with carbon in 5 (a)(ii).
b) A gas X has a vapour density of 32. It reacts with sodium hydroxide solution to form salt and water only. It decolourizes acidified potassium tetraoxomanganate (VII) solution and reacts with H2S to form sulphur. Using the information provided:
identify gas X; state two properties exhibited by X; give two uses of X.
c) Consider the following substances:
(1) sodium;
(2) lead (II) iodide;
(3) hydrogen;
(4) magnesium;
(5) oxygen.
Which of the substances
(i) conducts electricity?
(ii) is produced at the cathode during electrolysis of H2SO4(aq)?
(iii) corresponds to the molecular formula AB2 ?
(iv) is an alkaline earth metal?
d) (i) Define the term salt.
(ii) Mention two types of salt.
(iii) Give an example of each of the salts mentioned in 5(d)(ii) above.
e) In a neutralization reaction, dilute tetraoxosulphate (VI) acid completely reacted with sodium hydroxide solution.
(i) Write a balanced equation for the reaction.
(ii) How many moles of sodium hydroxide would be required for the complete neutralization of 0.50 moles of tetraoxosulphate (VI) acid?
6.
Note: There is nothing like Neco chemistry Expo online. Neco Ssce candidates are to desist from patronizing online fraudsters / vendors who says they can provide such services as they are not real.
Neco Chemistry Objective Questions 2023.
1.
2.
3.
4.
NOTE: There is nothing like Neco chemistry expo online. Do not be deceived by fraudsters posing fake NECO chemistry answers on the internet.
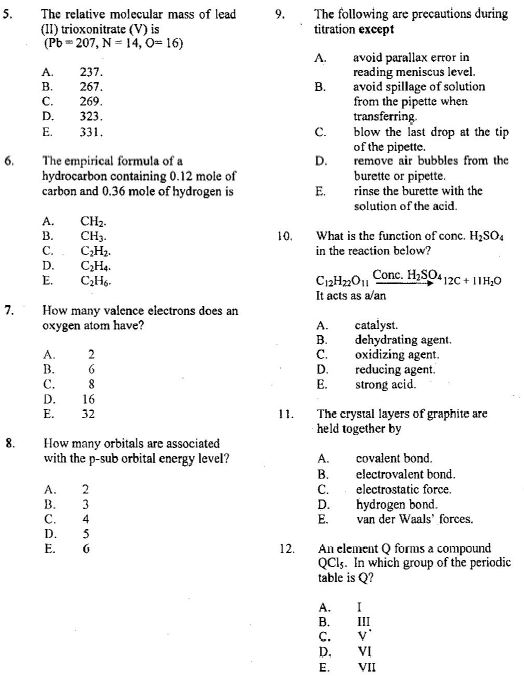
13. At a particular temperature and pressure, 15.0 g of CO2 occupy 7.16 liters. What is the volume of 12.0 g of CH4 at the same temperature and pressure?
A.
14.
Keep following this page. If you have any questions, endeavour to use the comment box below…

Are the questions legitimate
It was very useful
Please add me up
Can i get 2022 neco chemistry theory…
Lovely
This is very good
You are in point but not at all will people read there is expo
Can I get chemistry practical question of 2023?
We should all read our books & not rely on expo
Why u con dey this site
Today chemistry answers
Yes I agree wholeheartedly agree with you.
So what are u doing here
U are cross checking ur work nonsense
Lmao😭😂😂
I hope the questions are not fake
I need to join this group so that I can excel in my exam. So lovely. Thanks.
I want to join this group
I really need to join this group
thanks
I need the Neco 2021 questions and answers
Thanks
How about the answers
Obj and theory answers chemistry pls
Objective pls
How can I join please
I loved to join this group,this website is great, thanks so much
These chemistry questions above, the correct ones for neco 2021?
I wish to joined dis group
For chemistry obj n essay
please i need neco 2022 answers
I just love unn-edu.info. I wrote Agric exams neco today main paper and can you imagine that some of the questions they posted here came out today . So I advise anything that is posted here should be given utmost attention.
Thanks
Oyebode precious read your books, stop looking for expo
Where are the solution for those questions above.
The answer for this